Product regulatory review 2022 and view 2023
An overview of essential changes in selected areas of product and environmental law for non-food consumer products
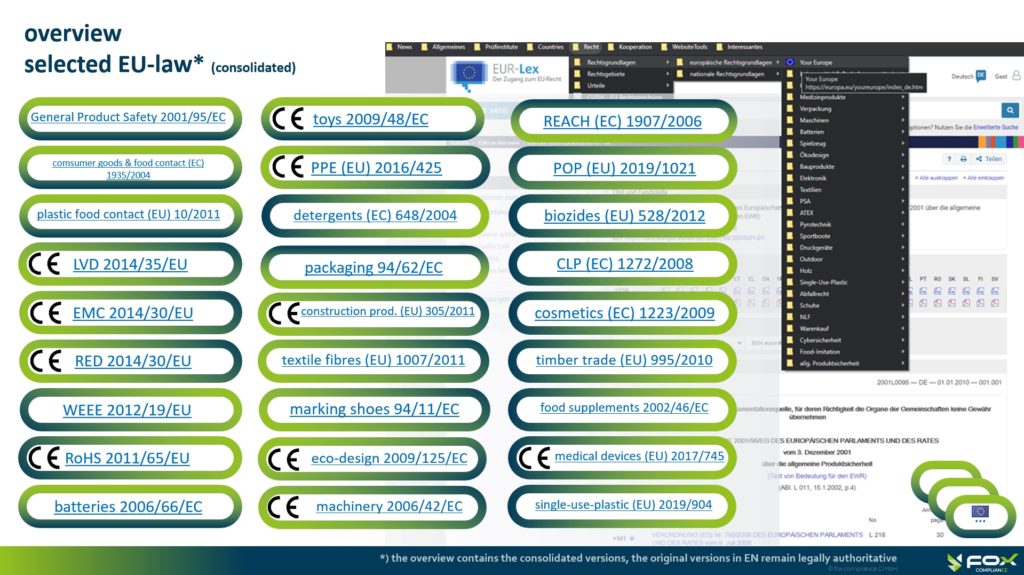
Overview
In view of the dynamic changes and complexity with which product law is currently developing, the overview presented, cannot claim to be complete. Rather, it is intended to give economic operators a first orientation and to be understood as a reference in the context of their “legal monitoring” of the legal minimum requirements during placing on the market or making available non-food consumer products of all kinds in Europe.
Due to the Corona pandemic (SARS-CoV-2 pandemic), the EU Commission also had difficulties in pushing forward its product regulatory agenda. The ambitious 2023 Commission work programme, with new legislative initiatives and the REFIT program to amend older legislation, can be viewed here.
The paradigm shift that has been underway for some time is recognizable here, in that Brussels is now increasingly turning to the instrument of a directly applicable EU regulation. This is gratifying in that within the harmonized goods market of the EU, legal provisions are also being subject to greater harmonization level. At the same time, however, the EU member states are being further restricted in their own national legal options. Overall, however, this development should have a positive effect on the EU-wide marketing and provision of products.
In the area of extended producer responsibility (EPR), on the other hand, it is already becoming apparent that the EU’s harmonization efforts are reaching their limits – more on this later.
General product safety – GPSR
On 21.12.2022, the final compromise text of the agreement in the EU trialogues (between the Parliament, the Council and the Commission) on the proposal for a new General Product Safety Regulation (GPSR) was published in the legislative procedure 2021/0170/COD with the document ST 16312 2022 INIT. This is a major step closer to publication in the Official Journal of the European Union. The GPSR will replace the General Product Safety Directive 2001/95/EC (GPSD) as well as the lesser-known Directive 87/357/EEC on products that can be confused with foodstuffs, thus providing far-reaching regulation for all consumer products. A more in-depth presentation of the upcoming innovations is reserved for a separate article. Also the other EU language versions will be published in the next days, under the document link mentioned above. A correspondence table at the end of the linked compromise text provides a first overview.
Every economic operator, whether he is a product-responsible manufacturer, importer or authorized representative, but also distributors, electronic marketplaces and fulfillment service providers, is advised to deal in detail with this regulation and the already valid Market Surveillance Regulation (EU) 2019/1020. The GPSR Regulation, which will then be directly applicable in all EU member states, could then come into force in the middle of next year 2024.
Chemical product safety – REACH
The REACH Regulation (EC) 1907/2006, which is decisive for chemical safety or material compliance, also received several updates in the form of amendments in 2022. A total of five different amendments have ensured that the EU’s “chemicals code” will continue to keep pace with scientific and technical progress in legislative terms.
For example, 24 substances are currently under evaluation – on a list maintained by the European Chemicals Agency (ECHA), whose development must be kept in mind for its medium-term product design. The Community Rolling Action Plan (CoRAP) is a tool used by the European Union (EU) to prioritize the risk assessment of substances that are already on the market and not yet fully listed under REACH. The CoRAP is updated annually and contains a list of substances that will be evaluated in the coming years by ECHA as the European scientific authority. This is to ensure that the most hazardous substances are identified and prioritized for risk assessment and risk management measures.
The draft provides for an annual update of the CoRAP and covers the three subsequent years 2023-2025. It includes substances suspected of endangering human health or the environment. Substance evaluation is the process under Articles 44 – 48 REACH, with the aim of clarifying potential risks from the use of these substances. The current draft CoRAP contains a total of 24 substances, including 6 new substances compared to the predecessor CoRAP 2022-2024; 5 substances are scheduled for evaluation in 2023, and 19 are split for evaluation in 2024 and 2025. Distributors must not disregard Annex XVII and draw practical consequences for their portfolio from the legal changes.
The POP Regulation (EU) 2019/1021 sometimes receives little attention in practice or is simply overlooked. This legal act is the implementation of the Stockholm Convention on Persistent Organic Pollutants within the EU and was supplemented for the sixth time in 2022.
Here, Annex I in particular should be reviewed for bans or limits, or Annex II for restrictions of substances in relation to many consumer products. Prominent substances in plastics in articles are e.g. short-chain chlorinated paraffins (SCCP), here the limit is 1500 ppm or polychlorinated biphenyls (PCB) in electrical appliances (ban by 31.12.2025). Hexabromocyclododecane (HBCD / HBCDD) is also still highly problematic in practice, e.g. in Styrofoam, and has been regulated with a limit of (only) 100 ppm.
Classification and labeling of chemicals
The classification and labeling of chemicals according to CLP Regulation (EC) 1272/2008 is also under examination.
The GHS (Globally Harmonized System) is a United Nations system for identifying hazardous chemicals and informing users of their hazards. Developed at the UN level, the GHS does not have immediate legal effect. It must first be made binding through implementation in the national legislation of individual states or communities of states. In Europe, this is done by the CLP Regulation. The amendments to the 17th ATP (Adaptation to Technical Progress) Regulation (EU) 2021/849 (application deadline: 17.12.2022) and 18th ATP Regulation (EU) 2022/692 (application deadline: 01.12.2023) must be taken into account.
In addition, a public consultation process is currently underway (here) from 20.12.2022 – 05.03.2023 regarding the revision of EU legislation on classification, labeling and packaging of chemicals. It is speculated that the EU will make its own classifications that go beyond the GHS.


Toys – TSD
Directive 2009/48/EC has been amended twice with legal effect in 2022.Here, too, the issue of converting the directive into a regulation is under discussion. Corresponding basic considerations can be found in the EU working paper SWD/2020/0287 final.
EPR area
(extended producer responsibility)
Contrary to what the generic term might suggest, the EPR is an obligation that only the economic actors responsible for the product have to fulfil. In short, the term “manufacturer” refers to those distributors in the respective EU member state who sells the B2C product to the end customer. These companies are then also in the focus of the respective national legislature, so they must make registrations in national registers in every legal area and then address quantity reports of the quantities they have sold there. With a Europe-wide sale of battery-powered electronics in end-customer packaging, there are therefore more than 100 registration obligations for companies across all countries.
The integration of EPR specialists, who provide strategic and/or operational support (make the respective quantity notifications) in fulfilling the legal obligations, is a good idea here.
Packaging
Packaging Directive 94/62/EC
In the area of European packaging law, the change in strategy, away from an EU directive to an EU regulation, is also being implemented. The necessary steps can be seen under the procedure 2022/0396(COD). The result is the proposed regulation COM(2022) 677 final, which will replace the previous EU directive 12 months after the new EU regulation enters into force.
Batteries and accumulators
The Battery Directive 2006/66/EC will be replaced by a regulation. The related procedure can be followed under 2020/0353/COD. New obligations will be imposed on economic operators that go beyond the existing requirements. Popularly overlooked in the context, but not included in the procedure, is Regulation (EU) 1103/2010 on rules for the indication of capacity on secondary (rechargeable) portable batteries and accumulators and on automotive batteries and accumulators.
Disposal of waste electrical and electronic equipment – WEEE
The Directive on Waste Electrical and Electronic Equipment 2012/19/EU WEEE was not adapted in 2022. However, the EU Commission conducted an evaluation to assess the progress and further development of this area of law with the public consultation from 06.10.2022 to 03.11.2022, which can be viewed here. The subject of frequent criticism was the lack of an effective mechanism to identify EPR free riders, because electrical and electronic equipment (EEE) supplied to the EU from third countries has so far been disregarded.
Overall, the EU still leaves much potential for harmonization in the area of EPR in terms of effective resource and recycling management.
In all three sub-areas, it is not creating a uniform category catalog with which the economic actors could work in a meaningful way. This catalog could also be the basis for a future European register and thus also the cornerstone for effective control by the competent authorities and ultimately significantly simplify the enforceability of the law. Instead, the EPR regulations are spreading nationally like a patchwork quilt, accompanied by national labels (Triman, sorting instructions in the national language, Info-Tri, Tidyman, recycling codes, Green Dot and much more). These processes are diametrically opposed to the basic idea of a single market for goods and should be addressed by the EU Commission. Unfortunately, neither the draft for the European battery regulation nor its counterpart in the packaging sector contains a better organizing European hand.
Chemical requirements for waste electrical and electronic equipment – RoHS
Directive 2011/65/EU RoHS for Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment has been legally adapted to scientific and technical progress once in 2022, with Annex III in particular regarding the use and transition periods of mercury in light bulbs likely to be of interest.
The Circular Economy Action Plan (CEAP), which counts electronics as a core product value within value chains, estimates that WEEE remains one of the fastest growing waste streams in the EU, with current annual growth rates of 2%.
Not least for this reason, the EU Commission had conducted a public consultation (see here) on the revision of Directive 2011/65/EU. In particular, the cumbersome and partly very non-transparent procedure in which exceptions and extensions of transitional periods are defined and published is often criticized. It is still being discussed whether the area of RoHS should be regulated as an independent instrument in the form of an amended directive, as a new regulation or as part of the REACH Regulation (EC) 1907/2006.
Electrical safety – LVD
Amendments to Directive 2014/35/EU regarding emergency procedures for conformity assessment are expected through procedure 2022/0280/COD.
Electromagnetic safety – EMC
Amendments to Directive 2014/30/EU are expected in relation to emergency procedures for conformity assessment through procedure 2022/0280/COD.
Radio equipment – RED
Directive (EU) 2022/2380 amending the RED Directive 2014/53/EU was published in the Official Journal of the European Union on December 7, 2022. The legislative procedure in the already completed procedure 2021/0291/COD can be viewed. It establishes USB Type-C technology as the standard charging port for the corresponding categories or classes of radio equipment.
Here, too, changes are to be expected with regard to emergency procedures for conformity assessment through procedure 2022/0280/COD.
Eco-design
A new Eco-design Framework Regulation is to replace the current framework legislation and thus Directive 2009/125/EC. The proposal COM(2022) 142 final states that significant changes are to be planned and introduced, leading in particular to more ecologically sustainable products. In particular, the digital product passport (DPP) should be mentioned here. The horizontal scope of application is also to be significantly extended beyond electrical products to include, for example, textiles. The procedure can be viewed under 2022/0095/COD.
Within the existing legal framework under the Eco-design Framework Directive 2009/125/EC, numerous implementing regulations are changing by tightening energy efficiency requirements and introducing new product groups covered by implementing measures. Electrical goods, textiles, furniture, steel, cement, and chemicals have been identified as potential product target groups. Thus, the existence of harmful chemicals will also be given greater consideration in the future. The overriding goal remains to reduce the environmental impact of products that are relevant to energy consumption, taking into account their entire life cycle. A selection of product groups and deadlines can be viewed here.
Single-use plastic – SUP
At this point, it is worth recalling the various implementation deadlines (addressed to the Member States, but affecting economic operators) of Article 17 of Directive (EU) 2019/904. This is visible in society, for example, in the packaging for fast food from which can be consumed immediately on the spot or taken away as a take-away dish. Thus, various obligations are introduced in the context of extended producer responsibility in the circular economy law. The Single-Use Plastic Fund Act (government draft: EWKFondsG), which was passed by the German Bundestag on 02.11.2022, sent to the Bundesrat and will apply from 2025, is, after the amendment of the national packaging law, the EWKVerbotsV and the EWKKennzV, the last national building block for the implementation of the SUP in the fight against single-use plastic products and the environmental problems they cause. The new law serves to establish a single-use plastic fund, which will be administered by the Federal Environment Agency (UBA). The UBA estimates that the 56,000 manufacturers of single-use plastic products affected will be held fiscally responsible and thus cover the costs of public and other waste disposal companies.
Food contact materials – FCM
Work is currently underway to revise EU regulations on food contact materials (FCMs). It is still unclear whether this will remain a mere revision or whether the process will even result in a recast of the entire legal framework under Regulation (EC) 1935/2004.
A public consultation on this is still running for a few days until 11.01.2023 and can be viewed here.
Regulation (EU) 10/2011 on plastic materials and articles intended to come into contact with food is also being revised. For this purpose, the drafts of the 16th / 17th / 18th amendment are available. The adoption of amendment 16 is very advanced and includes the tightening of the specific migration limits (SMLs) for plasticizers/phthalates as well as the lowering of the SML totals for DEHP, DBP, BBP and DIBP, the removal of the entry for wood flour and additions of some other substances. Amendment 17 will entail some adjustments due to Regulation (EU) 2022/1616 on recycled plastic materials and articles intended to come into contact with food. Amendment 18 will likely include specific migration limits for titanium dioxide and styrene, although the ECJ ruling (not yet final) ref. T-279/20 of 23.11.2022 (summary here) on classification under CLP Regulation (EC) 1272/2008 as carcinogenic and the scientific procedures that will now follow will certainly still have to be taken into account.
An initiative is also planned for the Ceramics Directive 84/500/EEC, last adapted in 2005, to be transferred to a new regulation at the end of 2023; the consultation on this can be viewed here. Here, the considerations of the revision of the entire legal framework are likely to play a role.
Textile (fiber) labeling
With Regulation (EU) 1007/2011, textile labeling tends to lead a rather unnoticed existence. Regularly the subject of consultation, it forms the minimum regulatory requirements for the labeling of numerous consumer products. Legally convoluted and with many exceptions and tolerances extremely user-unfriendly, this regulation (according to information from the UBA to the author) will probably also undergo a revision in the next two years. However, the subject of the update is completely unclear to date. It would be nice to have a clear legal scope and simplification for the economic actors (e.g. inclusion of this information in the planned digital product passport in the field of eco-design).
Medical devices – MDR
As can be seen from a press release of the EU Commission from 06.01.2023, the transitional periods from the old MDD Directive 93/42/EEC are now to be extended by an adjustment of the current Regulation (EU) 2017/745. At the meeting of the Council (Employment, Social Policy, Health and Consumer Affairs) on 09.12.2022, the EU health ministers called on the Commission to quickly submit a proposal to extend the transition period in the regulation on medical devices. The European Parliament and the Council will now negotiate on the proposal. The press release mentioned above is available here.
Machinery
A new Machinery Regulation has been adopted and will replace the current Machinery Directive 2006/42/EC, publication expected in the first half of 2023. The course of the procedure and the final version will be available under 2021/0105/COD.
Timber regulation – EU TR
The EU TR Regulation (EU) 995/2010 and its implementing regulation on due diligence schemes (EU) 607/2012 are about to be replaced by a new regulation on products associated with deforestation and forest degradation in supply chains. The process is underway under 2021/0366/COD and a compromise text was submitted for adoption on 21.12.2022 with document ST 16298 2022 INIT. After an 18-month transition period, in addition to wood products, cattle, cocoa, coffee, palm oil, soy and rubber will now also be regulated and risk assessed for legal origin through a DDS (due diligence system) before being placed on the market.
For the timber trade regulatory area, the following tariff codes are noted:
4402 wood charcoal, 4404 hoopwood, 4405 wood wool, 4417 tools with wooden components, 4420 4421 other articles of wood, including 4421 20 coffins pulp and paper of chapters 47 and 48 of the Combined Nomenclature, and 4900 Printed books, newspapers, pictures and other products of the printing industry, manuscripts, typescripts and plans.
The practical implementation of this should also be interesting, with which one should prove the geodata of the concrete area of the origin of the goods. The newly introduced differentiation in the legal definitions between primary forest, other forested areas and naturally regenerating forest also requires closer examination, as does the planned exemption from due diligence obligations for SMEs if a due diligence declaration has already been submitted in accordance with Article 31.
Supply Chain Due Diligence Act
(DE)
Here, companies must in any case keep an eye on developments in the Corporate Sustainability Due Diligence Directive (CSDDD) and amending the (Whistleblower) Directive (EU) 2019/1937). An adaptation of the national LkSG in Germany will most likely be the result, with both tightening or simplification of sustainable supply chain management conceivable depending on the outcome of the negotiations. The progress of the proceedings can be viewed at 2022/0051/COD.
Artificial intelligence – AI
Proposal COM (2021) 206 final on a regulation laying down harmonized rules for artificial intelligence (AI). Procedural history: 2021/0106/COD. As in other sectoral product regulations, a conformity assessment procedure along with EU declaration of conformity and CE marking is foreseen.
Brexit
Finally, a note on the use of the CE marking and ɜ-marking (reversed epsilon for aerosols) in the United Kingdom (UK).
As reported by the British government, this will now be accepted until 31.12.2024, so that companies can use it as a replacement or alongside the UKCA marking. Admittedly, compliance with the legal minimum standards must still be documented and proven upon request.
Further updates and more detailed information on individual regulatory areas will be published here or at www.foxcompliance.eu.

fox compliance GmbH · Germany
hello@foxcompliance.eu
www.foxcompliance.eu